The TricValve® system is a transcatheter bicaval valve replacement, an investigational device designed to prevent undesirable backflow of blood from the heart.
The unique system design includes two valves that are implanted in the right atrium using a minimally invasive delivery system. Your doctor places a thin tube through a vein in your leg to reach your heart and perform the procedure. This is not an open-heart surgery procedure.
TricValve® is a transcatheter therapy for people with severe TR. It is commercially available in Europe, Brazil, and several other countries outside of the U.S. and has been granted “breakthrough device” designation by the U.S. Food and Drug Administration (FDA) to expedite review.
As you may know from discussing your condition with your doctor, the tricuspid valve separates the right lower chamber (ventricle) from the right upper chamber (atrium) of the heart. Tricuspid regurgitation occurs when this valve doesn’t close properly and causes blood to leak backward.
If you have severe TR, you may experience:
Due to advanced age and other health concerns in these patients, doctors often consider the risk of surgical complications too high. That’s why many symptomatic patients are not referred for surgery or are deemed inoperable. The same is often true for the few approved minimally invasive treatment options. TricValve is currently the ONLY treatment option specifically designed to treat a wider range of patients.
If you have moderate to severe TR, you aren’t alone. The condition affects 1.6 million people in the U.S. Unfortunately, people with TR face a challenging future.
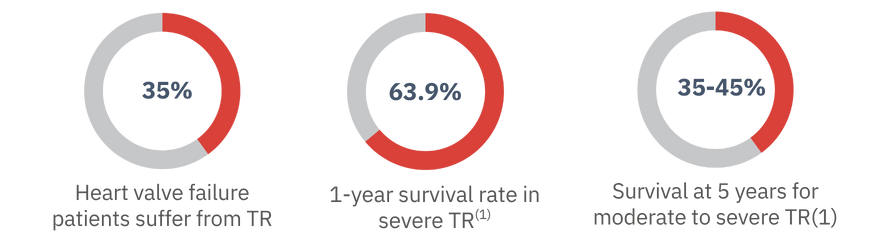
Many patients with severe TR in the U.S. can’t be treated with surgery or existing procedures due to the risks of complications. However, people elsewhere have another option: TricValve, a safe and minimally invasive therapy. TricValve has been successfully implanted in over 1,000 patients in various countries, but it has not yet been approved for use in the United States. The TRICAV Trial seeks to change that and give TR patients in the U.S. hope for improved quality of life.
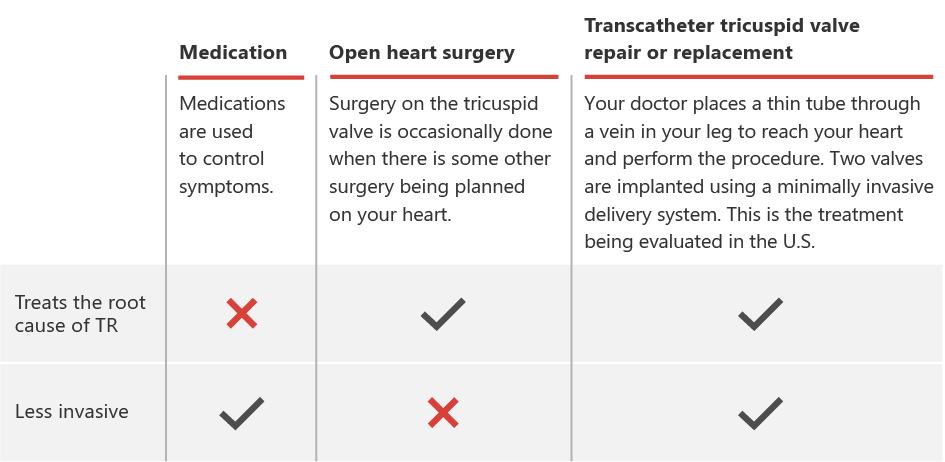
The TricValve procedure is a non-surgical option used to improve blood flow for patients with severe TR and significant RHF symptoms, utilizing its unique bicaval valves. During this procedure, a cardiologist uses a minimally invasive catheter delivery system to position self-expanding valves, just inside the right atrium.
This effectively reduces caval reflux (i.e. backward flow of blood into the IVC caused by tricuspid regurgitation) and has been shown to increase cardiac output in previous trials. The valves do this by reducing backward (or regurgitant) blood flow in patients with severe TR. As a result, patients experience less blood pooling in their extremities, reduced RHF symptoms, and improved overall condition.

The goal of the trial is to get FDA approval for this treatment and ensure commercial availability in the US for severe TR patients who are considered surgically inoperable and unsuitable for approved tricuspid repair or replacement for improving their quality of life.
Learn more about the TRICAV II Pivotal Trial at ClinicalTrials.gov.
The Local Heart Team will determine if you’re a good candidate for the study. They will:
If you meet the eligibility requirements, you will be randomly assigned to receive either:
If you’re enrolled in the TRICAV II trial, your study team will consist of several experts.
They will meet with you to review the TricValve procedure, explain the risks, and discuss the benefits of the procedure and what to expect after it. Then, the team members will ensure your condition is optimally medically managed before the procedure is performed.
This portion of the study is especially important. The team will need to do the following:
These visits are crucial as they provide information that will determine the study’s results — thereby helping others who are struggling with TR and RHF.
For details about the trial, visit NCT06458907 at ClinicalTrials.gov.
Learn more about the TRICAV Trial at ClinicalTrials.gov.
To be enrolled in the TRICAV Trial of the TricValve device, you must meet eligibility requirements.
Most participants will be:
No, you are not required to participate. Your doctor may recommend it as an option for you based on your medical history. This indicates that you may benefit from it, but you can choose not to participate.
The severe tricuspid valve regurgitation you are experiencing is causing a backflow of blood into your body, creating symptoms of fatigue, shortness of breath, swelling, and reduced quality of life. The TricValve procedure is intended to prevent the backflow and reduce the symptoms you’re currently experiencing.
No, it is not. TricValve device is placed using a catheter in a safe, non-surgical femoral vein puncture procedure.
Yes, you can. Having a pacemaker or implantable defibrillator doesn’t prevent participation in the study.
If you want to learn more about the TricValve product and the TRICAV-II Pivotal Trial, your cardiologist is an excellent source of information. They can answer questions like:
CAUTION – Investigational device. Limited by Federal (or United States) law to investigational use. This device is not available for marketing or commercial sale in the United States.
P & F USA, Inc. is the sponsor of the TRICAV-II Pivotal Trial.